
A total of 16 procedures were performed in the 15 patients.

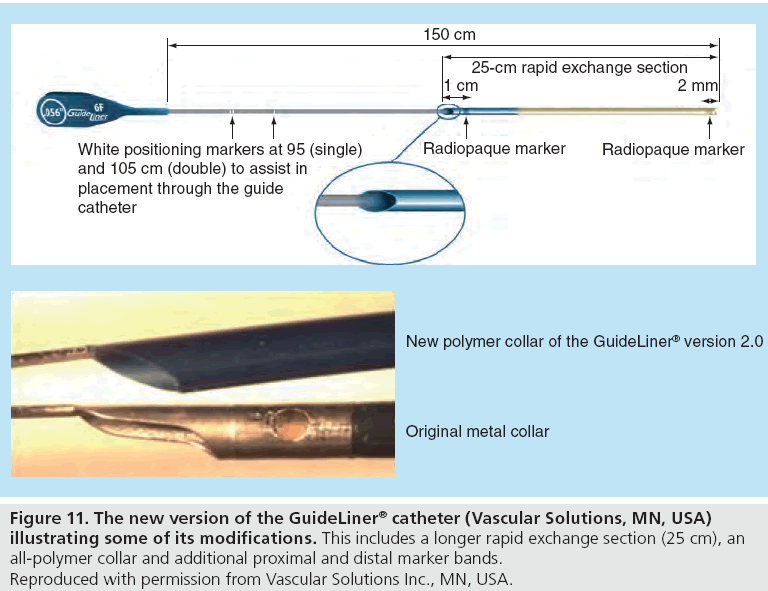
Subjects and MethodsĪll consecutive cases (15 in total 12 males and 3 females) of percutaneous coronary intervention (PCI) by a radial approach where the GuideLiner was used were prospectively included from January 1 to December 31, 2013. The aim of the present study was to describe the efficacy and safety of the use of this type of device in the treatment of complex lesions with the radial approach based on the initial experience at our center. Among these techniques are guide catheter extension devices like the GuideLiner® catheter (Vascular Solutions Inc.) that permits deep intubation and provides greater support and coaxiality. There are several techniques, such as the use of guide catheters with high passive back-up against the opposite aortic wall, active intubation of the guide, stiffer wires, anchoring or a ‘buddy wire', which can increase the strength of backup and support in complex cases. Hence, new percutaneous coronary strategies maximizing the efficacy, while minimizing the risk of complications, are needed. Theoretically, such patients are a group that may particularly benefit from the radial approach from a security point of view. Patients with this type of lesion have not only an increased risk of vascular complications but also a greater comorbidity burden. Nevertheless, the strength of support offered by the radial approach is significantly lower than that for femoral access, which implies an extra difficulty in the percutaneous treatment of complex coronary lesions. Ĭurrently, the radial approach is the most widely used in our setting due to its efficiency and low complication rate, in spite of the development of useful femoral closure devices. The presence of calcification, marked tortuosity or treatment of total chronic occlusions and the difficulty in the use of angioplasty balloons and stents lead to a considerable failure of stent deployment (approximately 3%) or to the development of other complications. The percutaneous treatment of complex coronary lesions is still a challenging problem, especially when using the transradial approach. Only one minor complication was recorded. GuideLiner was particularly helpful when using the transradial approach. Conclusion: Use of the GuideLiner was an effective and safe technique for the percutaneous treatment of complex coronary lesions in which the adequate progress of angioplasty devices had failed. A type B dissection of a proximal left circumflex artery was the only periprocedural complication. Unsuccessful cases were a chronic total occlusion and a diffusely diseased left anterior descendant artery. Results: Of the 16 angiographic procedures, 14 (87.5%) were successful (stent deployment in 13 cases and a drug-eluting balloon in 1 case). The indication for the use of GuideLiner was a difficulty to advance and properly position a stent through a tortuous and/or calcified artery despite using high-support guide catheters or other useful techniques. Sixteen consecutive procedures (in 15 patients 12 males and 3 females) were evaluated. The indication for its use, efficacy and periprocedural complications were determined. The transradial approach was used in all cases. Materials and Methods: The clinical, angiographic and procedural data of percutaneous coronary interventions where GuideLiner was used during 2013 were collected. The GuideLiner is as easy to insert as a standard rapid-exchange balloon catheter and has quickly become a routine part of my angioplasty practice.Objective: The aim of this study was to describe our initial experience with the GuideLiner® catheter (Vascular Solutions Inc.) in the transradial treatment of complex lesions. "Furthermore, the soft and very flexible tip will often cross tortuous disease where a stent gets stuck, enabling delivery of stents and other equipment directly to the target lesion. "Deep intubation of the GuideLiner catheter within a soft 6-F guide provides better backup support and is less traumatic than using stiff 7- and 8-F guides that were previously required in complex disease," commented Douglas Fraser, MD. In Europe, the GuideLiner received CE Mark clearance and was launched in October. Vascular Solutions will launch the device in the United States this month. The device is a coaxial "mother-and-child" guide extension with rapid exchange convenience that provides backup support and selective deep intubation in challenging interventions.Īccording to the company, the GuideLiner catheter will be available in 6-, 7-, and 8-F sizes as part of Vascular Solutions' specialty catheter product line. (Minneapolis, MN) announced that it has received 510(k) clearance from the US Food and Drug Administration to launch the GuideLiner catheter in the United States. November 9, 2009-Vascular Solutions, Inc.


 0 kommentar(er)
0 kommentar(er)
